valence electrons of bromine|how many valence electrons does bromine have : Clark The valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electrons without electron configuration. Knowing . Tingnan ang higit pa The name of the System ticket refers to how many numbers you can select. For example, with a System 10 ticket, you would select 10 numbers. Each game in Saturday Lotto has 6 numbers, so if you play a System 10, .
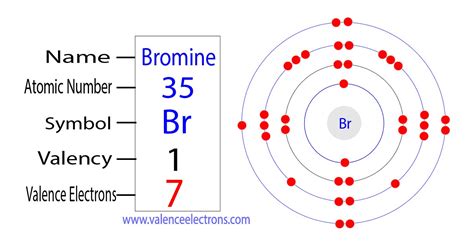
valence electrons of bromine,Bromine is a non-metallic element. The valence electrons are the total number of electrons in the last orbit(shell). The total number of electrons in the last shell after the electron configuration of bromineis called the valence electrons of bromine. The valence electrons determine the properties of the . Tingnan ang higit paThe valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electrons without electron configuration. Knowing . Tingnan ang higit paThe elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell during bond formation. . Tingnan ang higit paThe ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). The number of unpaired electrons in the last orbit of an element is the valency of that element. . Tingnan ang higit pa There are two ways to find the number of valence electrons in Bromine (Br). The first is to use the Periodic Table to figure out how many electrons Bromine has in its valence shell. .
Mar 23, 2023 Find the valences of bromine and other elements in a table based on the . Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. The valence electrons of bromine are seven and the . You will further explore the elements with its others significant properties. In chemistry, Bromine is a chemical element with its atomic number 35. It has the writing symbol as Br in the periodic table. .

Learn how to draw the Lewis structure of bromine, a diatomic molecule with seven valence electrons on each atom. Find out the molecular geometry, hybridisation, polarity and .Electron affinity The energy released when an electron is added to the neutral atom and a negative ion is formed. Electronegativity (Pauling scale) The tendency of an atom to . Titanium Valence Electrons. Vanadium Valence Electrons. Chromium Valence Electrons. Carbon Electron Configuration. Oxygen Electron Configuration. Manganese Valence Electrons. Helium . Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .
Explanation: Bromine is in family VII A. the same as Fluorine Chlorine. All members of the family have seven valance electron hence the name 7A. 7 only the electrons in the outmost shell are valance electrons.All but seven of the electrons in bromine are in lower shells Bromine is in family VII A. the same as Fluorine Chlorine. Method 2: From the Electron Configuration. If you want to find the valence electrons of bromine from its electron configuration, then you should know its electron configuration first. Now there are many methods to write the electron configurations, but here I will show you the easiest method, i.e by using Aufbau principle. Aufbau principle: .In the periodic table, bromine is a group VIIA element with seven electrons in its last shell. Therefore, the total number of valence electrons = 7(2) = 14. 2. Total electron pairs exist in the form of lone pairs and bonds. Total electron pairs are calculated by dividing the total valence electron count by two.

Generally, valence electrons can participate in the formation of chemical bonding, but core electrons cannot. While core electrons are not involved in bonding, they influence the chemical reactivity of an atom. The electron configuration of a oxygen atom is. O: 1s22s22p4 (1.9B.1) (1.9B.1) O: 1 s 2 2 s 2 2 p 4. which may be shorted.
Bromine atom has 7 valence electrons. For a main group element, a valence electron is an electron that has the highest principal quantum number, n. The electron configuration of Br is 1s² 2s²2p⁶ 3s²3p⁶ 4s²3d¹⁰4p⁵. The electrons with the highest quantum number are the 4s²4p⁵ electrons. Thus, Br has seven valence electrons.
The atomic number of bromine is 35, which means it has 35 electrons. Now it is possible to find the orbital notation of bromine very easily through electron configuration. That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5.
Valency of Bromine. Well, the exact valency of bromine remains 7 as its combining capacity. So,It has the 7 valence electrons in its outermost energy shell. Check out here for Bromine Valence Electrons and Bromine Valency (Br) Dot Diagram on this page. More information about Bromine also given. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable .
Bromine has seven valence electrons, and its valency is one. The given element is bromine and atomic number is 35. The symbol of the bromine element – Br. Electronic configuration: 1s22s22p63s23p64s23d104p5 or [Ar]4s23d104p5. The valency of bromine – +1. Bromine is a chemically reactive metal that is never pure in nature, as it reacts .
Bromine is a chemical element; . chlorine, and iodine. Bromine has the electron configuration [Ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all .
how many valence electrons does bromine haveElement Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element .valence electrons of bromine how many valence electrons does bromine have 3.1: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..
As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into the same group on Mendeleev's periodic table. Figure 11.1.1 11.1. 1: Periodic table by Dmitri Mendeleev, 1871.
There is only one element in bromine molecule. Bromine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of bromine atoms. valence electrons given by bromine atoms = 7 * 2 = 14; Total valence electrons = 14The diagram below shows the number of valence electrons (VE) for the main group elements. A periodic table showing how many valence electrons the main groups have. Group 1 = 1 valence electron Group 2 = 2 valence electrons Group 13 = 3 valence electrons Group 14 = 4 valence electrons Group 15 = 5 valence electrons Group 16 = .Solution. Verified by Toppr. Valence electrons found in the s and p orbitals of the highest energy. Bromine has an electron configuration of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5 the valence electrons are in the 4 s and 4 p orbitals giving Bromine 7 valence electrons. Was this answer helpful? the shielding experienced by nd or nf valence electrons electrons within same group shield 0.35; electrons within the lower groups shield 1.00; . Exercise \(\PageIndex{1}\): The Shielding of valence p Electrons of Bromine Atoms. What is the shielding constant experienced by a valence p-electron in the bromine atom?
valence electrons of bromine|how many valence electrons does bromine have
PH0 · why does bromine have 7 valence electrons
PH1 · valence electrons in o2
PH2 · valence electrons for all elements
PH3 · valence electron configuration for bromine
PH4 · list of valence electrons for each element
PH5 · how many valence electrons does bromine have
PH6 · how do you find valence electrons
PH7 · finding valence electrons in ions
PH8 · Iba pa